Brand: Upneeq
Upneeq
- Regular
- $168.00
- Sale
- $168.00
- Regular
- Unit Price
- per
This is a prescription product and only available to current patients of Bay Area Cosmetic Dermatology with an active prescription. Please use the 'Certify to Add to Cart' button. Once completed you will be able to then add the product to cart.
If you would like to schedule an appointment, you can book online here.
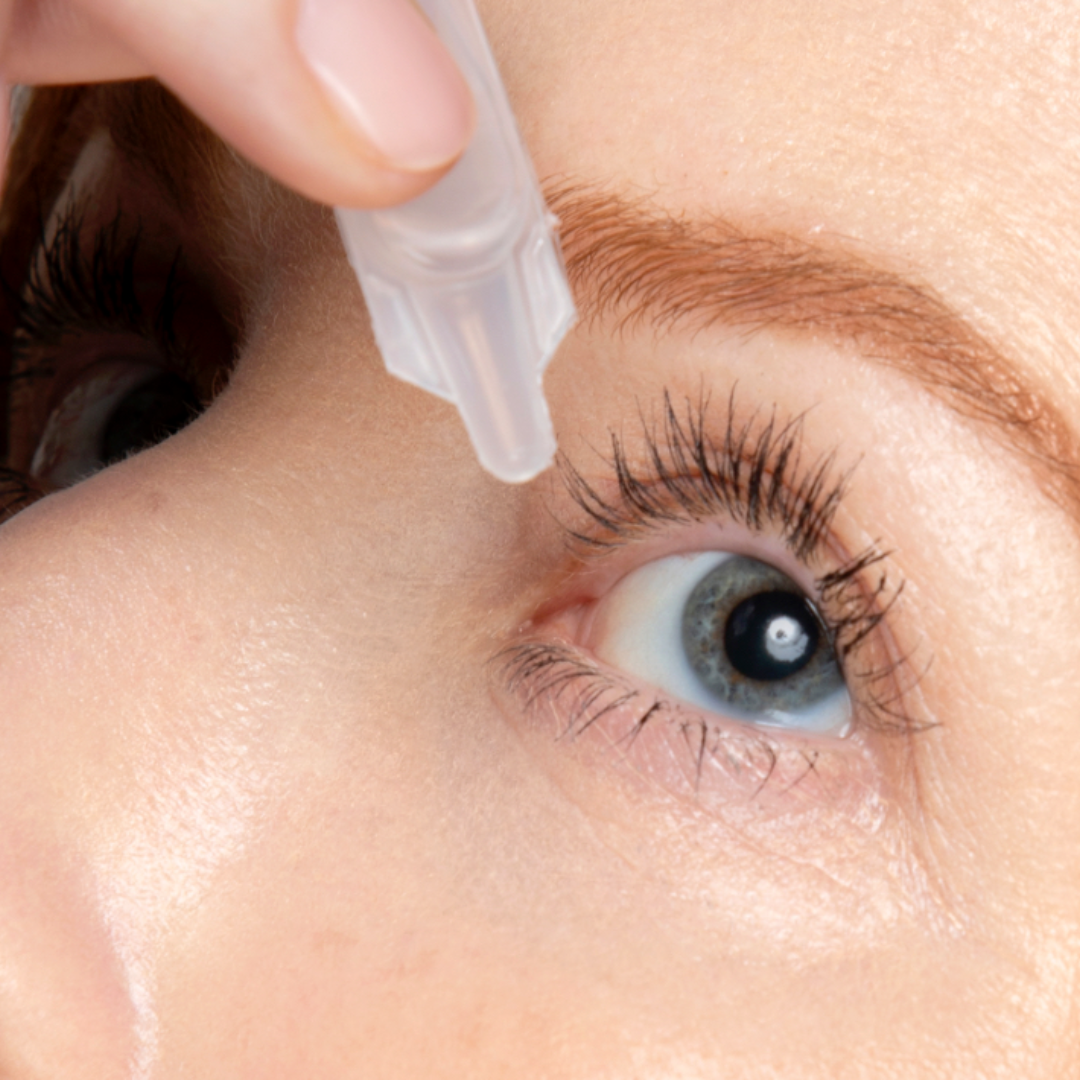
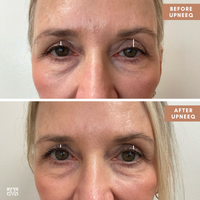
Imagine an eye opening Lift with a Daily Drop of Upneeq® (oxymetazoline hydrochloride ophthalmic solution), 0.1%. The only FDA-approved prescription eyedrop for acquired ptosis (low-lying lids) that lifts your upper eyelids to open your eyes.
This medication is used to treat a certain eyelid condition (acquired blepharoptosis) that causes drooping upper eyelid(s). It belongs to a class of drugs known as alpha agonists. Oxymetazoline works by making a certain eyelid muscle get shorter and tighter (contract).
In clinical trials, Upneeq helped patients with aquired ptosis see more - on the first day of treatment!
- 84% of patients had some level of improvement
- 74% of patients had more than a 50% impovement
- In one study, some patients saw a lift in their eyelids as fast as 5 minutes after the first dose
Upneeq is safe and well-tolerated.
- In clinical trials, Upneeq was proven to be safe and effective when used as directed.
- Common side effects (seen in 1-5% of patients) included eye inflammation, eye redness, dry eye, blurred vision, instillation site pain, eye irritation and headache.
UPNEEQ ® (oxymetazoline hydrochloride ophthalmic solution), 0.1% contains oxymetazoline hydrochloride, an alpha adrenoceptor agonist.
UPNEEQ ® is an aseptically prepared, sterile, nonpreserved ophthalmic solution.
Each mL of UPNEEQ ® (oxymetazoline hydrochloride ophthalmic solution) 0.1% contains 1 mg of oxymetazoline hydrochloride, equivalent to 0.09 mg (0.09%) of oxymetazoline free base. The ophthalmic solution contains the following inactive ingredients: calcium chloride, hydrochloric acid (used to adjust pH to 5.8 to 6.8), hypromellose, magnesium chloride, potassium chloride, sodium acetate, sodium chloride, sodium citrate, and water for injection.
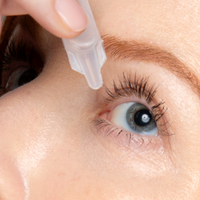
Step 1: Cut open the foil wrapper and remove the single-use vial.
Step 2: Apply one drop of Upneeq® in each affected eye as directed, once a day.
• Do not let the tip of the vial touch your eye or any other surface
• Vials should not be re-used after opening and should be thrown away after applying drop(s)
• If you wear contact lenses, remove them before applying Upneeq® eyedrops
• You may put them back in 15 minutes after applying Upneeq®
• If more than one topical ophthalmic drug is being used, the drugs should be administered at least 15 minutes between applications
Upneeq® should only be used as directed
• Upneeq® is designed to only be applied as an eyedrop.
• Each vial contains enough Upneeq® solution to allow for one drop in each affected eye.
• The solution should never be swallowed or ingested in any other way.
Do not use Upneeq® if you have glaucoma.
Storage requirements:
Upneeq® should be stored at 68°F-77°F (20°C-25°C) and should be protected from excessive heat.
• Keep out of reach of children